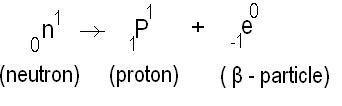What is the formula for number of nuclei present at any instant?
The Radioactive Decay law states that the number of nuclei undergoing decay per unit time is directly proportional to the number of unchanged nuclei present at that instant.
The formula for Radioactive Decay law is given as,
– (dN / dt) ∝ N
– (dN / dt) = λ N
So we can say that at a time t = 0 we have N0 radioactive nuclei present, substituting the current values in the above formula we get the number of radioactive nuclei present at time t.
So the formula for number of nuclei present at any instant is N = N0 e-λt where
N = number of atoms
λ = decay constant
N0 = number of nuclei when time is 0 (the initial quantity)
λ = decay constant
t = time
posted in Atoms, Molecules and Nuclei | Comments Off on What is the formula for number of nuclei present at any instant?